Katja Tolkkinen, University of Oulu

Osteoarthritis is a degenerative disease of the articular cartilage, and it is one of the most common reasons for disability. Articular cartilage is an essential part of the human body because it allows smooth and painless movement of the joint. It is the highly specialized, porous, gel-like connective tissue allowing heavy loads to be carried, but unfortunately in case of damage, it cannot heal on its own. Cartilage degradation causes pain, swelling, and inability to move and work, which cost a lot for society. Since osteoarthritis is often detected only in its end-stages, the common form of treatment is the joint replacement surgery, which is a major medical and economical problem. The degeneration of articular cartilage is directly related to the changes in its protein network, pore structures and water compartments. To prevent the disease from progressing to the irreversible stage, it is necessary to develop earlier and more efficient diagnostics and medicines for osteoarthritis, which requires basic understanding of macromolecular scale phenomena in cartilage matrix.
Nuclear Magnetic Resonance (NMR) is a phenomenon in which the atomic nucleus interacts with an external magnetic field by absorbing and re-emitting electromagnetic radiation. The use of non-invasive radiofrequency radiation enables study of opaque objects without damaging them. In NMR, nuclei that have the quantum property of spin possess a magnetic dipole moment which interacts with external magnetic fields. Like small bar magnets, the dipoles tend to turn parallel to external field. The external magnetic fields of NMR spectrometers are usually on the order of several teslas, and they are produced by large superconducting magnets. Traditional NMR experiments are based on flipping the magnetization of the spins from one direction to another by radiofrequency pulses, and finally detecting the relaxation of magnetization back to equilibrium while recording the NMR signal. Overall, NMR experiments can provide information on chemical, structural and dynamic properties of materials, and the importance of NMR is also emphasized by its application as clinical magnetic resonance imaging (MRI) which is one of the widely used diagnostic methods today.
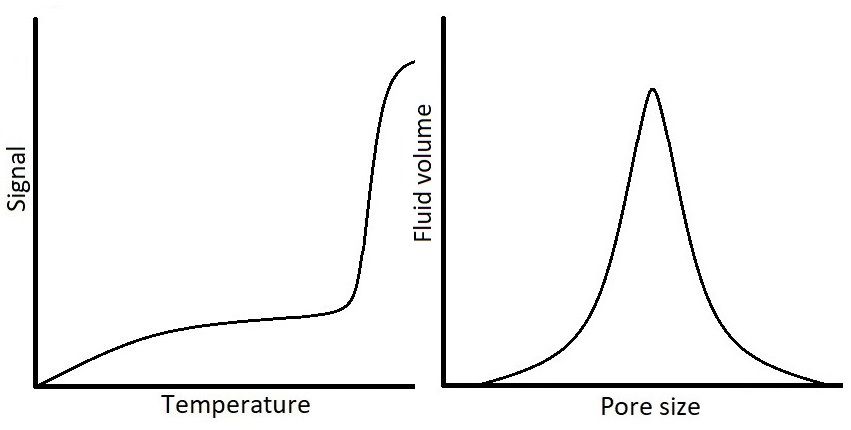
One example of important opaque materials is porous materials. Characterization of porous materials is of interest to several fields of science due to the vast presence of porous materials in nature, biological systems, industry, and technology. Because of the increasing use of porous materials in research, there is a need for methods capable of determining pore size distributions (PSD) of materials. Thermoporometry methods utilize the observation of solid-liquid phase transitions to measure pore sizes on the order of nanometers to micrometers. Nuclear Magnetic Resonance Cryoporometry (NMRC) is a thermoporometry method in which the detection of phase transition is based on the different NMR properties of solid and liquid. The method utilizes the Gibbs-Thomson equation according to which the melting point depression of pore-confined liquid is inversely proportional to the pore size. In other words, the smaller the pore, the lower the melting point compared to the bulk melting point of probed substance. In NMRC experiment, a sample is cooled down until all the liquid, including the liquid confined in pores, is frozen, and after that the sample is warmed up back to the room temperature in small temperature steps, while the NMR signal of the liquid is recorded. Since in NMRC experiment it is possible to record only the signal of liquid and dismiss the signal of solid, the experiment results in a cryoporometric melting curve from which the PSD can be computed by the Gibbs-Thomson equation.
NMRC is a versatile method since many solvents can be used as a cryoporometric probe fluids. The most common solvent is water, however, in biological materials water occurs usually as a saline solution, which possess challenges to thermoporometry. In saline, in addition to bulk melting and pore-related melting, there occurs a third phase transition called a eutectic phase transition. As water in saline starts to freeze, the NaCl concentration of remaining water increases, and the melting point of remaining saline gets lower until the solution reaches the eutectic temperature
where the remaining water and NaCl solidifies into a single component called hydrohalite (NaClH2O). From the cryoporometric point of view, when the sample containing saline is heated back towards the room temperature, the melting of this hydrohalite phase produces an additional liquid signal to the melting curve. Since the Gibbs-Thomson equation assumes that the liquid signal below the bulk melting point of 0⁰C is all related to liquid in pores, this causes problems in analysis of the PSD data which is derived from the melting curve. The development of the extended Gibbs-Thomson equation for saline solutions would open huge new possibilities for the thermoporometry and NMRC of porous biomaterials.
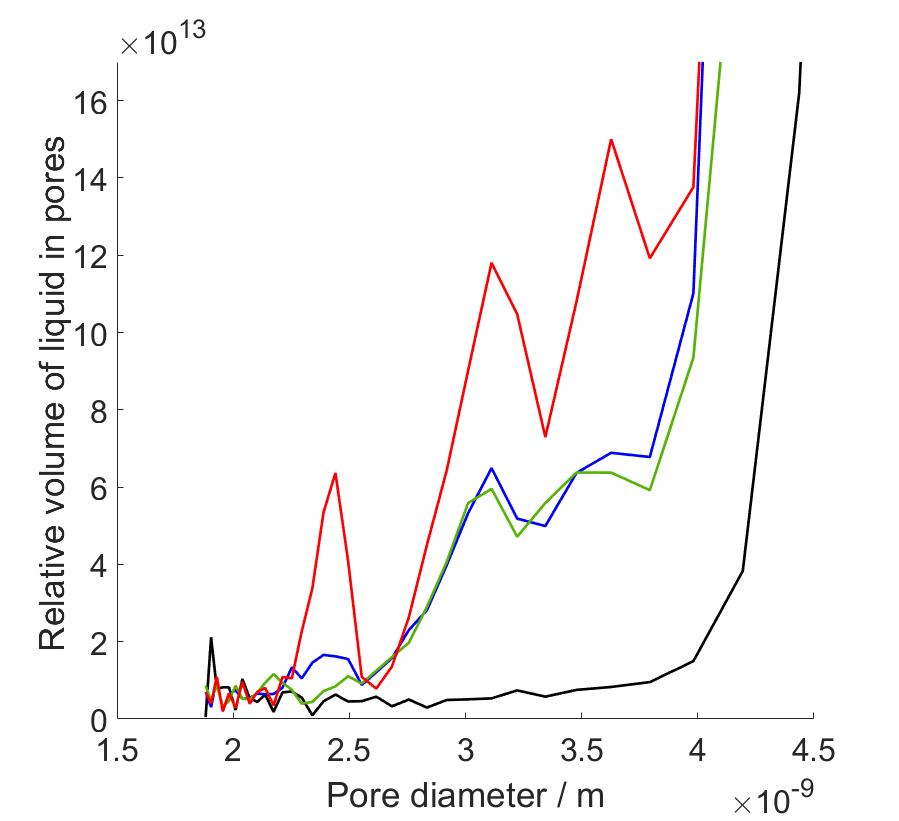
In my master’s thesis work, I carried out proton NMRC experiments on saline solutions, porous silica bead samples hydrated with water and saline, and protein hydrogels modelling the protein composition of articular cartilage, with the end goal of determining the PSDs and investigating protein-associated water in human articular cartilage. Since we know that saline cryoporometry is complex due to the eutectic phase transition and distribution of melting points related to change in NaCl concentration during melting, before the investigation of protein samples, we performed NMRC experiments on water, saline solutions and hydrated porous silica to observe the difference in melting behavior caused by saline. We used information obtained from this part to interpret the data of cartilage gels, since the proteins were immersed in saline to prevent the protein swelling. Our protein hydrogel samples contained varying concentrations of collagen and chondroitin sulphate (CS), the main protein components of cartilage extracellular matrix. The water in cartilage is either free to move or contained in the porous structures formed by the proteins. In my research, the hypothesis was that by increasing the protein concentrations, we could observe the increase in intensities of peaks in PSDs since there is more pore-related water in the pore network. In other words, the objective was to identify to which proteins cartilage water is associated with and to determine corresponding PSDs.
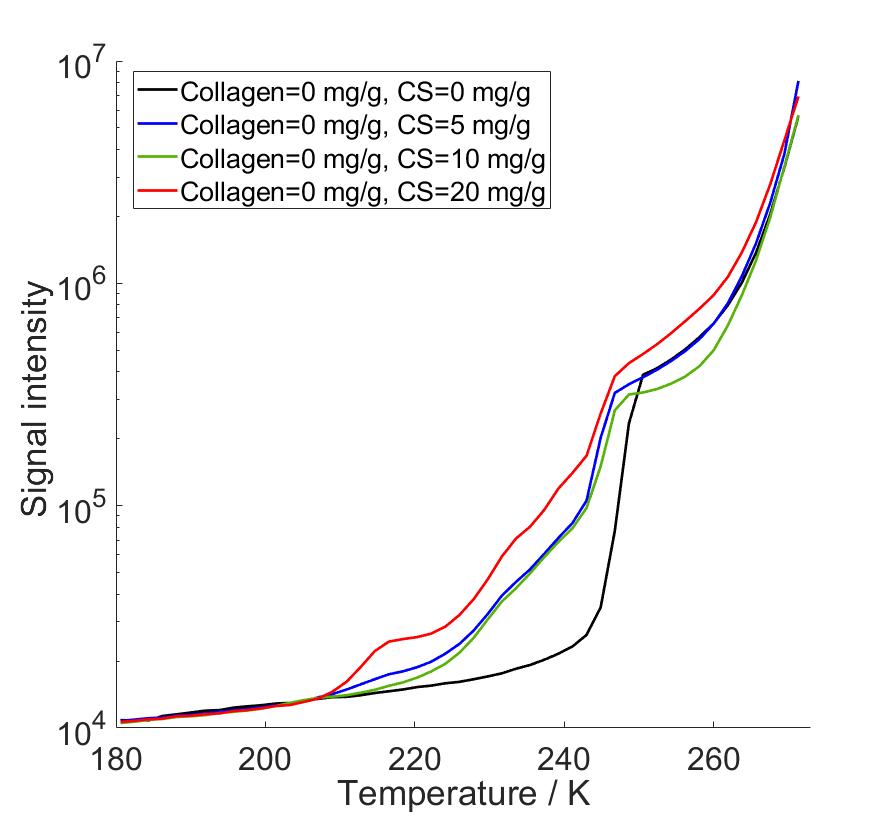
As a result, we observed that there occurs a eutectic phase transition in saline solutions, and saline as a solvent altered the calculated PSDs compared to water as a solvent. We observed that the eutectic phase transition produced a very high intensity peaks in PSD calculations in the same pore size range with actual pore diameters, covering part of the desired peaks. Due to that, we performed data analysis with two different methods, first with full temperature range data, and secondly changing the bulk melting point of the Gibbs-Thomson equation to observed eutectic temperature at which hydrohalite melting starts. In this way we were able to reduce non-pore-related data from our analysis. By combining the benefits of both analysis methods, we were able to identify PSD peaks increasing in intensity as both collagen and CS concentration increased. The corresponding pore diameters fell between 2-4 nm which are consistent with cartilage pore sizes of 2-6 nm determined in hydraulic permeability experiments in previous reports.
To understand how our PSD results can be utilized also for development of better diagnostics, we performed T2 relaxation time measurements with varying protein concentrations. T2 is related to diagnostics of osteoarthritis since it is one of the most used contrasts in clinical cartilage imaging. Our experiments showed that T2 relaxation times at room temperature were sensitive to the amount collagen but not to the amount of CS, which is consistent with previous reports. However, previous research show that T1ρ relaxation is sensitive for the changes in CS, making T1ρ complementary to T2. The relaxation times are directly related to pore sizes and for that reason, relaxation studies combined with pore size information can help to develop more sensitive and efficient cartilage imaging methods, and to find MRI contrast agents that would be able to be absorbed into the smallest pore structures in articular cartilage.
This work shows that NMR cryoporometry is a promising method for characterization of biomaterials, although the use of saline requires special data processing, and therefore data analysis methods need to be further developed. This study also shows that very simple protein models can provide a lot of new information about tissue as complex as articular cartilage. PSD studies of cartilage can provide understanding on the conditions of osteoarthritis and help in both medicine and imaging design. The development of MRI may provide earlier diagnosis for osteoarthritis, possibly even when cartilage is still recoverable. Altogether, NMR gives new insights into articular cartilage and its degenerative diseases.